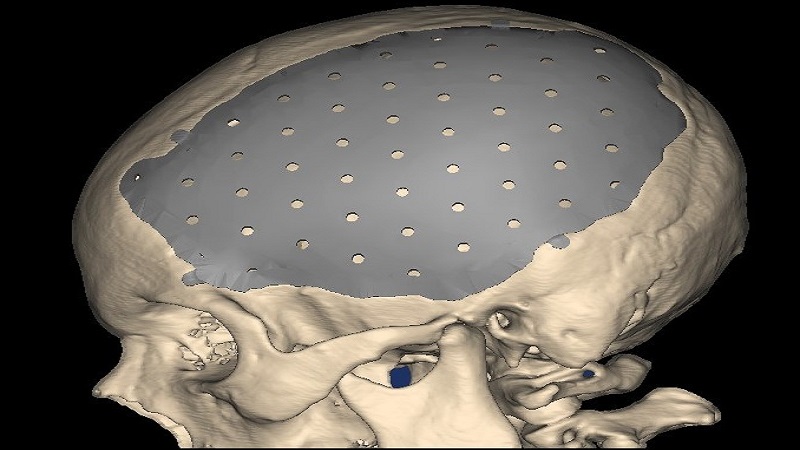
Local company BioArchitects was founded by young entrepreneur, Felipe Marques four years ago with investment in medical 3D Printing to harvest the technology. The company now uses metal 3D printing technology to create patient-specific, biocompatible implants that replace hard tissue and allows doctors to actually be able to see and manipulate a replica of what they will find when they operate. The BioArchitects also performs in field of medical training, simulated operations and prosthetics, with their titanium plate being first of its kind to be approved for use by the US Food and Drug Administration (FDA).