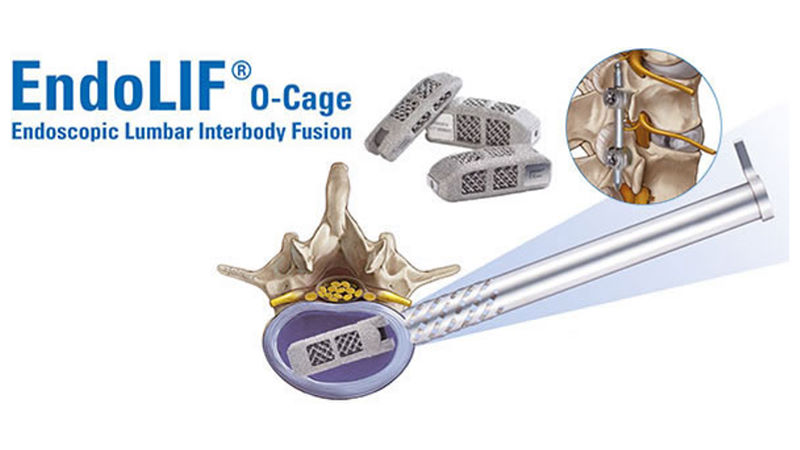
Joimax announced that they have received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in order to market their Endoscopic Lumbar Interbody Fusion (EndoLIF® On-Cage implant) in the U.S.

Joimax announced that they have received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in order to market their Endoscopic Lumbar Interbody Fusion (EndoLIF® On-Cage implant) in the U.S.