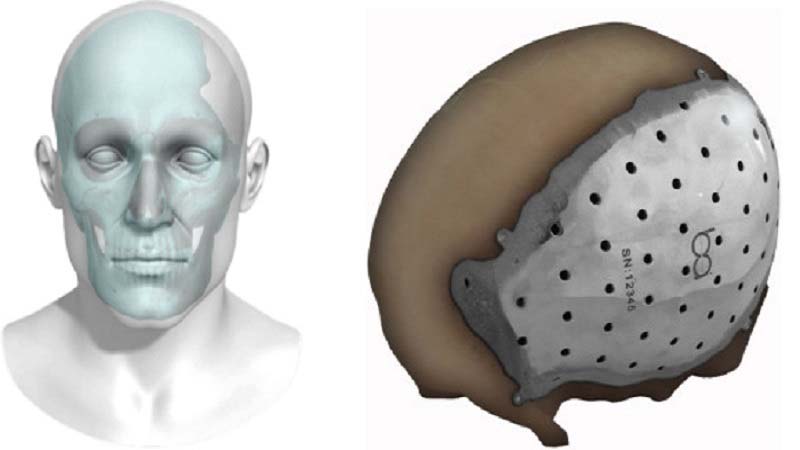
BioArchitects announced today the 510(k) clearance by the U.S. Food and Drug Administration – FDA, for the company’s 3D printed patient specific titanium cranial/craniofacial plate implant. Starting from CT scan or MRI of the affected area, the image is then imported into a highly sophisticated computer design program, which is used to create a template of the repair that becomes the model from which the 3D printer produces the titanium plate which is the exact fit for the defect.
 Medical 3D Printing & Bioprinting
Medical 3D Printing & Bioprinting