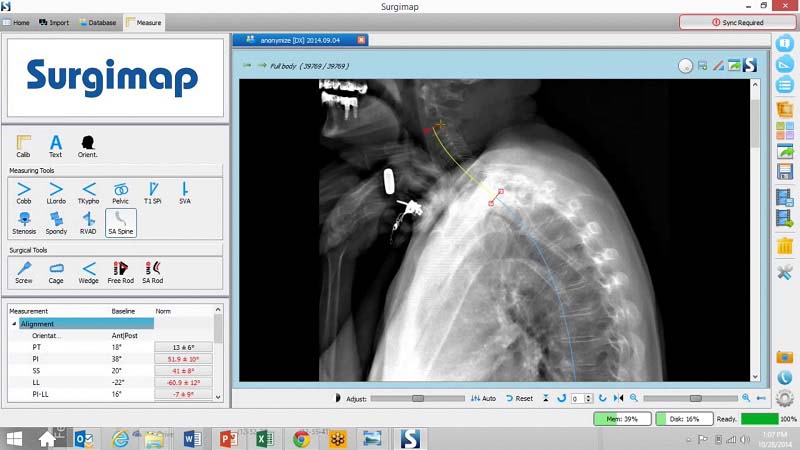
Medicrea, announced FDA approval of the first-ever patient-specific UNiD™Cervical rod for spine surgery, secured by their complementary PASS OCT® posterior cervical stabilization system. Being the only medical device company offering patient-specific implant solutions for spinal conditions, it rolled out it's first implantation of the UNiD™ Patient-Specific Cervical Rod in New York City on February 17,2016.
 Medical 3D Printing & Bioprinting
Medical 3D Printing & Bioprinting